Studying the effectiveness and safety of VYVGART
The VYVGART for IV infusion study explored changes in daily abilities and muscle weakness as well as safety in adults with anti-AChR antibody positive gMG.
The VYVGART Hytrulo for subcutaneous injection study compared antibody reduction—which is related to gMG symptoms—and safety in patients taking either VYVGART Hytrulo for subcutaneous injection or VYVGART for IV infusion.
The VYVGART for IV infusion study
The main goals of this study were to see how VYVGART for IV infusion improved daily abilities and reduced muscle weakness in patients taking VYVGART for IV infusion plus their current oral gMG treatment, compared to those taking placebo plus their current oral gMG treatment.
68% (44 of 65) of patients who added VYVGART for IV infusion to their current oral gMG treatment significantly improved their ability to perform daily activities, as measured by the Myasthenia Gravis Activities of Daily Living (MG-ADL) scale.*
Compared to 30% (19 of 64) of patients on placebo plus their current oral gMG treatment.
63% (41 of 65) of patients who added VYVGART for IV infusion to their current oral gMG treatment saw a significant reduction in muscle weakness, as measured by the Quantitative Myasthenia Gravis (QMG) scale.†
Compared to 14% (9 of 64) of patients on placebo plus their current oral gMG treatment.
The VYVGART for IV infusion study:
VYVGART was evaluated in a global study of adults with anti-AChR antibody positive gMG. The primary goals of the study were to examine effectiveness (improved daily abilities and reduced muscle weakness) and safety for FDA approval of VYVGART. The study examined the effectiveness and safety of VYVGART in 167 adults (18 years or older) with gMG. In addition to their current treatment, patients received either VYVGART or placebo.
*Defined as a decrease of 2 or more points on the MG-ADL scale, which measures the severity of 8 common symptoms of gMG, maintained for at least 4 weeks. A lower MG-ADL score means less severe symptoms.
†Defined as a decrease of 3 or more points on the QMG scale, which assesses muscle weakness based on 13 items, maintained for at least 4 weeks. A lower QMG score means less muscle weakness.
The VYVGART Hytrulo for subcutaneous injection study
In this study, VYVGART Hytrulo for subcutaneous injection and VYVGART for IV infusion had a similar average reduction in the harmful AChR antibodies that cause gMG symptoms.
Additional data from the VYVGART for IV infusion study
The following data points were not the main goals of the study and do not carry the same weight as the main goals of the study. No reliable conclusions can be drawn about the effectiveness or safety of VYVGART for IV infusion based on these data points. Talk to your neurologist if you have any questions.
MG-ADL data during the first treatment cycle
of patients who added VYVGART for IV infusion to their current oral gMG treatment were observed to have an MG-ADL score of 0 or 1, which means minimal or no symptoms, during at least 1 visit during their first treatment cycle
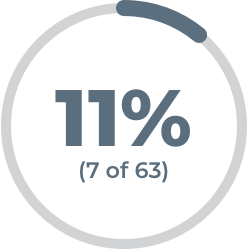
of patients on placebo plus their current oral gMG treatment were observed to have an MG-ADL score of 0 or 1, which means minimal or no symptoms, during at least 1 visit during their first treatment cycle
The MG-ADL scale is an assessment tool that measures the severity of 8 common symptoms of gMG. Each item is scored on a 4-point scale—a score of 0 means “normal,” and a score of 3 means “loss of ability.” The total score can range from 0 to 24 points, with a higher score showing more severe gMG.
MG-ADL data after 2 treatment cycles
of patients who added VYVGART for IV infusion to their current oral gMG treatment were observed to have an MG-ADL response by the time they completed a second treatment cycle‡
of patients on placebo plus their current oral gMG treatment were observed to have an MG-ADL response by the time they completed a second treatment cycle§
MG-ADL response was defined by a decrease of 2 or more points on the MG-ADL scale, maintained for 4 or more weeks.
‡44 of 65 patients on VYVGART for IV infusion had an MG-ADL response during treatment cycle 1. Of the 21 patients who didn’t have a response, 7 responded during the second treatment cycle.
§19 of 64 patients on placebo plus their current gMG treatment had an MG-ADL response during treatment cycle 1. Of the 45 patients who didn't have a response, 9 responded during the second treatment cycle.
Things to discuss with MY doctor before starting VYVGART Hytrulo or VYVGART
How to get VYVGART your way
Prefilled and can go where you go
Inject in your own space, whether that’s your kitchen table or a hotel room with a view. It is injected under your skin (subcutaneously). It takes about 20-30 seconds.||
Personalized one-on-one training
You and/or your caregiver will receive in-person training at home or in your doctor’s office until you're ready to inject on your own. You’ll also get a demonstration kit to help you practice.
VYVGART Hytrulo for subcutaneous injection and VYVGART for IV infusion can also be given at an infusion center, doctor’s office, or at home.¶
Talk to your doctor about your needs, preferences, coverage, and other factors to understand your options.
||Please see Patient Information. Follow appropriate administration steps and storage and handling guidance in the Instructions for Use. Monitor for signs and symptoms of an allergic reaction for at least 30 minutes after injection. If an allergic reaction occurs, you should seek medical attention.
¶In some cases, VYVGART Hytrulo for subcutaneous injection or VYVGART for IV infusion may be given at home by a trained nurse.
Broad access available for MY treatment#
#Based on published policies from Policy Reporter as of August 2024.
Getting started with VYVGART
VYVGART safety
Learn about safety and side effects for VYVGART for IV infusion and VYVGART Hytrulo for subcutaneous injection.
VYVGART dosing
Breaks between VYVGART treatment cycles are personalized to you. Find out how to work with your neurologist to make the most of your treatment plan.
Real Stories on VYVGART
This video series features real people who speak about the impact of living with anti-AChR antibody positive gMG—and their experiences with VYVGART.
Do not take VYVGART if you are allergic to efgartigimod alfa or any of the ingredients in VYVGART. Do not take VYVGART HYTRULO if you are allergic to efgartigimod alfa, hyaluronidase, or any of the ingredients in VYVGART HYTRULO. VYVGART or VYVGART HYTRULO can cause serious allergic reactions and a decrease in blood pressure leading to fainting.
Before taking VYVGART or VYVGART HYTRULO, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection or fever.
- have recently received or are scheduled to receive any vaccinations.
- have any history of allergic reactions.
- have kidney (renal) problems.
- are pregnant or plan to become pregnant. It is not known whether VYVGART or VYVGART HYTRULO will harm your unborn baby.
- Pregnancy Exposure Registry. There is a pregnancy exposure registry for women who use VYVGART or VYVGART HYTRULO during pregnancy. The purpose of this registry is to collect information about your health and your baby. Your healthcare provider can enroll you in this registry. You may also enroll yourself or get more information about the registry by calling 1-855-272-6524 or going to VYVGARTPregnancy.com
- are breastfeeding or plan to breastfeed. It is not known if VYVGART or VYVGART HYTRULO passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVGART or VYVGART HYTRULO can cause side effects which can be serious, including:
- Infection. VYVGART or VYVGART HYTRULO may increase the risk of infection. If you have an active infection, your healthcare provider should delay your treatment with VYVGART or VYVGART HYTRULO until your infection is gone. Tell your healthcare provider right away if you get any of the following signs and symptoms of an infection:
- fever
- chills
- frequent and painful urination
- cough
- pain and blockage of nasal passages
- wheezing
- shortness of breath
- sore throat
- excess phlegm
- nasal discharge
- Allergic reactions (hypersensitivity reactions). VYVGART or VYVGART HYTRULO can cause allergic reactions that can be severe. These reactions can happen during, shortly after, or weeks after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction with VYVGART or VYVGART HYTRULO:
- rash
- swelling of the face, lips, throat, or tongue
- shortness of breath
- trouble breathing
- low blood pressure
- fainting
An additional symptom of an allergic reaction with VYVGART HYTRULO can include hives.
- Infusion or injection-related reactions. VYVGART can cause infusion-related reactions. VYVGART HYTRULO can cause infusion or injection-related reactions. These reactions can happen during or shortly after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider if you have any of the following symptoms of an infusion or injection-related reaction:
- high blood pressure
- chills
- shivering
- chest, stomach, or back pain
The most common side effects of VYVGART or VYVGART HYTRULO include respiratory tract infection, headache, and urinary tract infection. An additional common side effect with VYVGART HYTRULO includes injection site reactions.
These are not all the possible side effects of VYVGART or VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is VYVGART® (efgartigimod alfa-fcab) for intravenous (IV) infusion and what is VYVGART HYTRULO® (efgartigimod alfa and hyaluronidase-qvfc) for subcutaneous injection?
VYVGART and VYVGART HYTRULO are both prescription medicines used to treat adults with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
It is not known if VYVGART or VYVGART HYTRULO is safe and effective in children.
Please see full Prescribing and Patient Information for VYVGART HYTRULO for subcutaneous injection and full Prescribing Information for VYVGART for IV infusion.
Dosage forms and strengths:
VYVGART Hytrulo is available as a single-dose subcutaneous injection containing: 200 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per prefilled syringe, or 180 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per vial. VYVGART is available as a single-dose injection for intravenous use containing 400 mg/20 mL of efgartigimod alfa-fcab per vial.
IMPORTANT SAFETY INFORMATION
Do not take VYVGART if you are allergic to efgartigimod alfa or any of the ingredients in VYVGART. Do not take VYVGART HYTRULO if you are allergic to efgartigimod alfa, hyaluronidase, or any of the ingredients in VYVGART HYTRULO. VYVGART or VYVGART HYTRULO can cause serious allergic reactions and a decrease in blood pressure leading to fainting.
Before taking VYVGART or VYVGART HYTRULO, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection or fever.
- have recently received or are scheduled to receive any vaccinations.
- have any history of allergic reactions.
- have kidney (renal) problems.
- are pregnant or plan to become pregnant. It is not known whether VYVGART or VYVGART HYTRULO will harm your unborn baby.
- Pregnancy Exposure Registry. There is a pregnancy exposure registry for women who use VYVGART or VYVGART HYTRULO during pregnancy. The purpose of this registry is to collect information about your health and your baby. Your healthcare provider can enroll you in this registry. You may also enroll yourself or get more information about the registry by calling 1-855-272-6524 or going to VYVGARTPregnancy.com
- are breastfeeding or plan to breastfeed. It is not known if VYVGART or VYVGART HYTRULO passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVGART or VYVGART HYTRULO can cause side effects which can be serious, including:
- Infection. VYVGART or VYVGART HYTRULO may increase the risk of infection. If you have an active infection, your healthcare provider should delay your treatment with VYVGART or VYVGART HYTRULO until your infection is gone. Tell your healthcare provider right away if you get any of the following signs and symptoms of an infection:
- fever
- chills
- frequent and painful urination
- cough
- pain and blockage of nasal passages
- wheezing
- shortness of breath
- sore throat
- excess phlegm
- nasal discharge
- Allergic reactions (hypersensitivity reactions). VYVGART or VYVGART HYTRULO can cause allergic reactions that can be severe. These reactions can happen during, shortly after, or weeks after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction with VYVGART or VYVGART HYTRULO:
- rash
- swelling of the face, lips, throat, or tongue
- shortness of breath
- trouble breathing
- low blood pressure
- fainting
An additional symptom of an allergic reaction with VYVGART HYTRULO can include hives.
- Infusion or injection-related reactions. VYVGART can cause infusion-related reactions. VYVGART HYTRULO can cause infusion or injection-related reactions. These reactions can happen during or shortly after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider if you have any of the following symptoms of an infusion or injection-related reaction:
- high blood pressure
- chills
- shivering
- chest, stomach, or back pain
The most common side effects of VYVGART or VYVGART HYTRULO include respiratory tract infection, headache, and urinary tract infection. An additional common side effect with VYVGART HYTRULO includes injection site reactions.
These are not all the possible side effects of VYVGART or VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is VYVGART® (efgartigimod alfa-fcab) for intravenous (IV) infusion and what is VYVGART HYTRULO® (efgartigimod alfa and hyaluronidase-qvfc) for subcutaneous injection?
VYVGART and VYVGART HYTRULO are both prescription medicines used to treat adults with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
It is not known if VYVGART or VYVGART HYTRULO is safe and effective in children.
Please see full Prescribing and Patient Information for VYVGART HYTRULO for subcutaneous injection and full Prescribing Information for VYVGART for IV infusion.
Dosage forms and strengths:
VYVGART Hytrulo is available as a single-dose subcutaneous injection containing: 200 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per prefilled syringe, or 180 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per vial. VYVGART is available as a single-dose injection for intravenous use containing 400 mg/20 mL of efgartigimod alfa-fcab per vial.