
Discover what to expect leading up to treatment, from navigating insurance to beginning your first cycle.
Caregiver Guide

Get self-care tips and education about VYVGART so you can continue to help support and advocate for your loved one.
VYVGART Co-pay Program brochure

Learn how you might pay as little as $0 for VYVGART and related administration costs through the Co-pay Program.*
*Eligible commercially insured patients may pay as little as $0 for VYVGART and may receive a maximum benefit of $25,000 per calendar year for their eligible out-of-pocket costs for the drug and drug administration. Persons residing in MA and RI are not eligible for financial assistance related to administration costs. Please see full Terms and Conditions.
Insurance and Coverage Guide

See how My VYVGART Path can help you navigate insurance and explore potential financial assistance programs.
VYVGART Dosing Guide

Understand dosing, including how regularly tracking symptoms can inform your personalized breaks.
Subcutaneous Self-Injection Guide

Learn about the process of self-injection with the VYVGART Hytrulo prefilled syringe, from preparation to disposal.
In-Office Subcutaneous Injection Guide

Learn about dosing and how to prepare for your VYVGART Hytrulo subcutaneous injection, as well as what to expect afterwards.
Infusion Guide

Learn what to expect when you receive VYVGART for intravenous (IV) infusion, from preparation to aftercare.
MG-ADL assessment tool
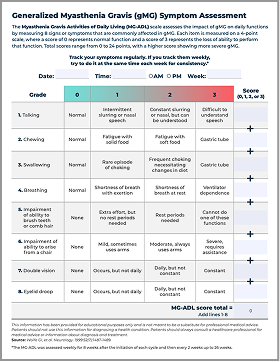
Regularly track your symptoms with the Myasthenia Gravis Activities of Daily Living (MG-ADL) tool—it may help you and your doctor plan your treatment cycles.
Understanding your gMG symptoms: Vision and eye symptoms
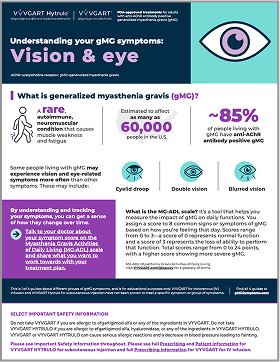
Learn about the vision and eye-related symptoms gMG can cause and get answers to commonly asked questions about them.
Understanding your gMG symptoms: Face, mouth, and throat symptoms

See how gMG can affect the face, mouth, and throat—and get tips to discuss these symptoms with your doctor.
Understanding your gMG symptoms: Breathing problems
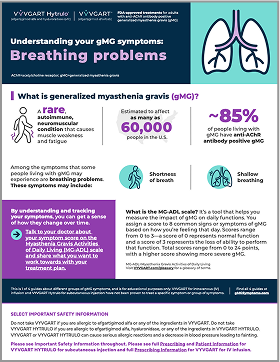
Get to know what breathing problems gMG can cause and how they may impact you.
Understanding your gMG symptoms: Limb weakness
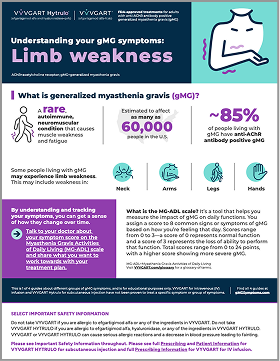
Understand how gMG might impact the strength of your limbs so you can better track your symptoms over time.
Conversation Guide for Family, Friends, and Loved Ones

Get help having productive conversations with family and friends where you can define and ask for the support you might need.
Patient emergency contact card

Use this card to share treatment information and key contacts in the case of an emergency.
Explore more support for your treatment journey
Nurse Case Managers
Get to know our NCMs, who will provide personalized support throughout your journey.
Cost and coverage
Find resources and information that may help you navigate coverage for VYVGART.
My VYVGART Path interactive tour
Map out your journey by exploring support at every step of your treatment.
Do not take VYVGART if you are allergic to efgartigimod alfa or any of the ingredients in VYVGART. Do not take VYVGART HYTRULO if you are allergic to efgartigimod alfa, hyaluronidase, or any of the ingredients in VYVGART HYTRULO. VYVGART or VYVGART HYTRULO can cause serious allergic reactions and a decrease in blood pressure leading to fainting.
Before taking VYVGART or VYVGART HYTRULO, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection or fever.
- have recently received or are scheduled to receive any vaccinations.
- have any history of allergic reactions.
- have kidney (renal) problems.
- are pregnant or plan to become pregnant. It is not known whether VYVGART or VYVGART HYTRULO will harm your unborn baby.
- Pregnancy Exposure Registry. There is a pregnancy exposure registry for women who use VYVGART or VYVGART HYTRULO during pregnancy. The purpose of this registry is to collect information about your health and your baby. Your healthcare provider can enroll you in this registry. You may also enroll yourself or get more information about the registry by calling 1-855-272-6524 or going to VYVGARTPregnancy.com
- are breastfeeding or plan to breastfeed. It is not known if VYVGART or VYVGART HYTRULO passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVGART or VYVGART HYTRULO can cause side effects which can be serious, including:
- Infection. VYVGART or VYVGART HYTRULO may increase the risk of infection. If you have an active infection, your healthcare provider should delay your treatment with VYVGART or VYVGART HYTRULO until your infection is gone. Tell your healthcare provider right away if you get any of the following signs and symptoms of an infection:
- fever
- chills
- frequent and painful urination
- cough
- pain and blockage of nasal passages
- wheezing
- shortness of breath
- sore throat
- excess phlegm
- nasal discharge
Allergic reactions (hypersensitivity reactions). VYVGART or VYVGART HYTRULO can cause allergic reactions that can be severe. These reactions can happen during, shortly after, or weeks after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction with VYVGART or VYVGART HYTRULO:
- rash
- swelling of the face, lips, throat, or tongue
- shortness of breath
- trouble breathing
- low blood pressure
- fainting
An additional symptom of an allergic reaction with VYVGART HYTRULO can include hives.
- Infusion or injection-related reactions. VYVGART can cause infusion-related reactions. VYVGART HYTRULO can cause infusion or injection-related reactions. These reactions can happen during or shortly after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider if you have any of the following symptoms of an infusion or injection-related reaction:
- high blood pressure
- chills
- shivering
- chest, stomach, or back pain
The most common side effects of VYVGART or VYVGART HYTRULO include respiratory tract infection, headache, and urinary tract infection. An additional common side effect with VYVGART HYTRULO includes injection site reactions.
These are not all the possible side effects of VYVGART or VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is VYVGART® (efgartigimod alfa-fcab) for intravenous (IV) infusion and what is VYVGART HYTRULO® (efgartigimod alfa and hyaluronidase-qvfc) for subcutaneous injection?
VYVGART and VYVGART HYTRULO are both prescription medicines used to treat adults with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
It is not known if VYVGART or VYVGART HYTRULO is safe and effective in children.
Please see full Prescribing and Patient Information for VYVGART HYTRULO for subcutaneous injection and full Prescribing Information for VYVGART for IV infusion.
Dosage forms and strengths:
VYVGART Hytrulo is available as a single-dose subcutaneous injection containing: 200 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per prefilled syringe, or 180 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per vial. VYVGART is available as a single-dose injection for intravenous use containing 400 mg/20 mL of efgartigimod alfa-fcab per vial.
IMPORTANT SAFETY INFORMATION
Do not take VYVGART if you are allergic to efgartigimod alfa or any of the ingredients in VYVGART. Do not take VYVGART HYTRULO if you are allergic to efgartigimod alfa, hyaluronidase, or any of the ingredients in VYVGART HYTRULO. VYVGART or VYVGART HYTRULO can cause serious allergic reactions and a decrease in blood pressure leading to fainting.
Before taking VYVGART or VYVGART HYTRULO, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection or fever.
- have recently received or are scheduled to receive any vaccinations.
- have any history of allergic reactions.
- have kidney (renal) problems.
- are pregnant or plan to become pregnant. It is not known whether VYVGART or VYVGART HYTRULO will harm your unborn baby.
- Pregnancy Exposure Registry. There is a pregnancy exposure registry for women who use VYVGART or VYVGART HYTRULO during pregnancy. The purpose of this registry is to collect information about your health and your baby. Your healthcare provider can enroll you in this registry. You may also enroll yourself or get more information about the registry by calling 1-855-272-6524 or going to VYVGARTPregnancy.com
- are breastfeeding or plan to breastfeed. It is not known if VYVGART or VYVGART HYTRULO passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVGART or VYVGART HYTRULO can cause side effects which can be serious, including:
- Infection. VYVGART or VYVGART HYTRULO may increase the risk of infection. If you have an active infection, your healthcare provider should delay your treatment with VYVGART or VYVGART HYTRULO until your infection is gone. Tell your healthcare provider right away if you get any of the following signs and symptoms of an infection:
- fever
- chills
- frequent and painful urination
- cough
- pain and blockage of nasal passages
- wheezing
- shortness of breath
- sore throat
- excess phlegm
- nasal discharge
Allergic reactions (hypersensitivity reactions). VYVGART or VYVGART HYTRULO can cause allergic reactions that can be severe. These reactions can happen during, shortly after, or weeks after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction with VYVGART or VYVGART HYTRULO:
- rash
- swelling of the face, lips, throat, or tongue
- shortness of breath
- trouble breathing
- low blood pressure
- fainting
An additional symptom of an allergic reaction with VYVGART HYTRULO can include hives.
- Infusion or injection-related reactions. VYVGART can cause infusion-related reactions. VYVGART HYTRULO can cause infusion or injection-related reactions. These reactions can happen during or shortly after your VYVGART infusion or VYVGART HYTRULO injection. Tell your healthcare provider if you have any of the following symptoms of an infusion or injection-related reaction:
- high blood pressure
- chills
- shivering
- chest, stomach, or back pain
The most common side effects of VYVGART or VYVGART HYTRULO include respiratory tract infection, headache, and urinary tract infection. An additional common side effect with VYVGART HYTRULO includes injection site reactions.
These are not all the possible side effects of VYVGART or VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is VYVGART® (efgartigimod alfa-fcab) for intravenous (IV) infusion and what is VYVGART HYTRULO® (efgartigimod alfa and hyaluronidase-qvfc) for subcutaneous injection?
VYVGART and VYVGART HYTRULO are both prescription medicines used to treat adults with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
It is not known if VYVGART or VYVGART HYTRULO is safe and effective in children.
Please see full Prescribing and Patient Information for VYVGART HYTRULO for subcutaneous injection and full Prescribing Information for VYVGART for IV infusion.
Dosage forms and strengths:
VYVGART Hytrulo is available as a single-dose subcutaneous injection containing: 200 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per prefilled syringe, or 180 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per vial. VYVGART is available as a single-dose injection for intravenous use containing 400 mg/20 mL of efgartigimod alfa-fcab per vial.













