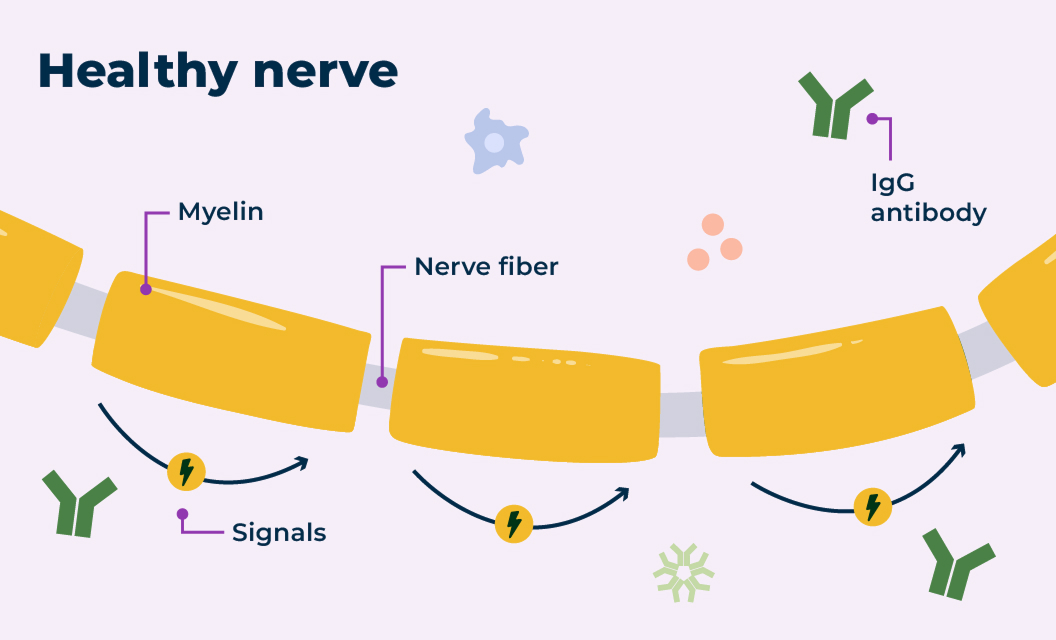
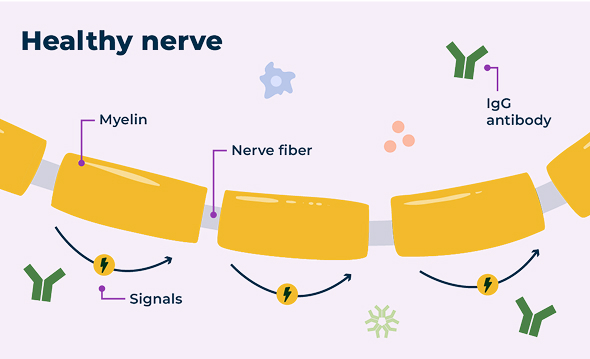
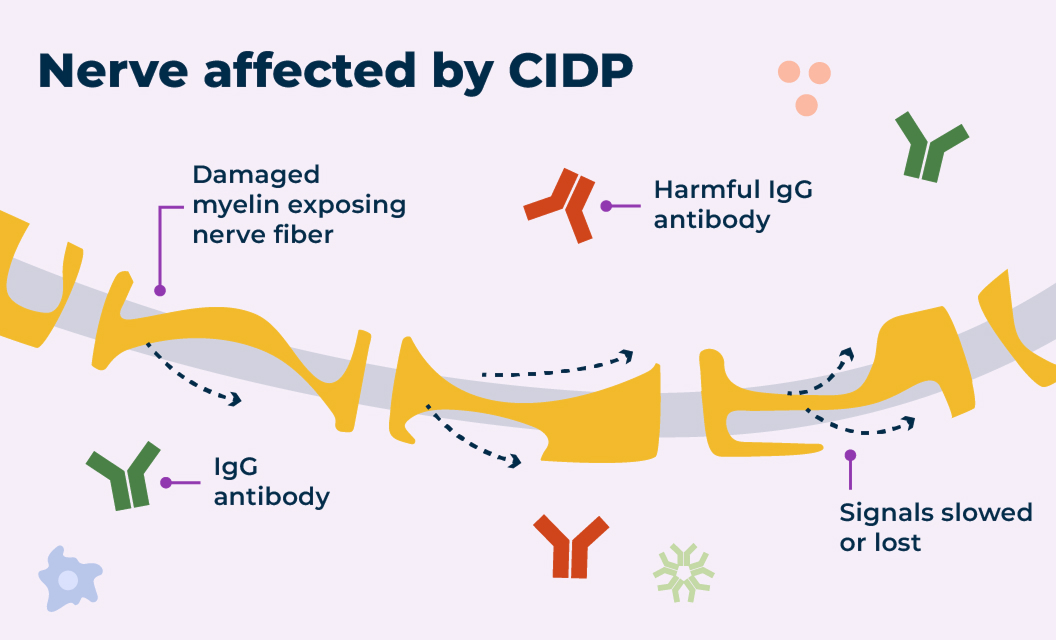
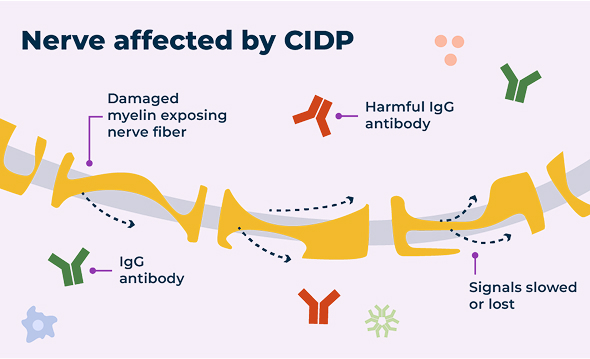
In a healthy nervous system, nerve cells send signals to and from the brain using electrical currents. The protective layer around the nerve, called myelin, helps those signals travel efficiently.
Common symptoms people with CIDP experience
Chronic inflammatory demyelinating polyneuropathy can cause people to experience symptoms in their arms and legs. This can make it hard for them to do daily activities. Possible symptoms include:
About CIDP
Although the complete cause of CIDP is not fully understood, recent studies suggest that, in some people, harmful IgG antibodies may be involved in damaging the myelin.
What is the role of harmful IgG antibodies?
In some people with CIDP, the immune system doesn’t just produce helpful IgG antibodies that work to protect you—it also mistakenly creates harmful IgG antibodies that work against you.
Nerves with and without CIDP
For some people with CIDP, harmful IgG antibodies that remain in the body too long can cause damage to the myelin.
What is the impact of harmful IgG over time?
Damage to the myelin over time makes it harder for signals to travel quickly and efficiently along the nerve. That means signals to and from the arms and legs may be slowed or in some cases lost completely, which is why some people experience CIDP symptoms. Symptoms like muscle weakness and tingling or numbness can affect your ability to perform daily tasks. The damage may be debilitating and, in some cases, irreversible.
IgG=immunoglobulin G.
Learn more about VYVGART Hytrulo
How VYVGART Hytrulo works
Discover how VYVGART Hytrulo is specifically designed to help cause a reduction in the number of IgG antibodies, including harmful IgG antibodies.
Self-injection with the VYVGART Hytrulo prefilled syringe
The first and only once-weekly self-injection for CIDP that takes about 20-30 seconds.*
*Please see Patient Information. Follow appropriate administration steps in the Instructions for Use. Monitor for signs and symptoms of an allergic reaction for at least 30 minutes after injection. If an allergic reaction occurs, you should seek medical attention.
Do not take VYVGART HYTRULO if you are allergic to efgartigimod alfa, hyaluronidase, or any of the ingredients in VYVGART HYTRULO. VYVGART HYTRULO can cause serious allergic reactions and a decrease in blood pressure leading to fainting.
Before taking VYVGART HYTRULO, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection or fever.
- have recently received or are scheduled to receive any vaccinations.
- have any history of allergic reactions.
- have kidney (renal) problems.
- are pregnant or plan to become pregnant. It is not known whether VYVGART HYTRULO will harm your unborn baby.
- Pregnancy Exposure Registry. There is a pregnancy exposure registry for women who use VYVGART HYTRULO during pregnancy. The purpose of this registry is to collect information about your health and your baby. Your healthcare provider can enroll you in this registry. You may also enroll yourself or get more information about the registry by calling 1-855-272-6524 or going to VYVGARTPregnancy.com
- are breastfeeding or plan to breastfeed. It is not known if VYVGART HYTRULO passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVGART HYTRULO can cause side effects which can be serious, including:
- Infection. VYVGART HYTRULO may increase the risk of infection. If you have an active infection, your healthcare provider should delay your treatment with VYVGART HYTRULO until your infection is gone. Tell your healthcare provider right away if you get any of the following signs and symptoms of an infection:
- fever
- chills
- frequent and painful urination
- cough
- pain and blockage of nasal passages
- wheezing
- shortness of breath
- sore throat
- excess phlegm
- nasal discharge
- Allergic reactions (hypersensitivity reactions). VYVGART HYTRULO can cause allergic reactions that can be severe. These reactions can happen during, shortly after, or weeks after your VYVGART HYTRULO injection. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction:
- rash
- swelling of the face, lips, throat, or tongue
- shortness of breath
- hives
- trouble breathing
- low blood pressure
- fainting
- Infusion or injection-related reactions. VYVGART HYTRULO can cause infusion or injection-related reactions. These reactions can happen during or shortly after your VYVGART HYTRULO injection. Tell your healthcare provider if you have any of the following symptoms of an infusion or injection-related reaction:
- high blood pressure
- chills
- shivering
- chest, stomach, or back pain
The most common side effects of VYVGART HYTRULO include respiratory tract infection, headache, urinary tract infection, and injection site reactions.
These are not all the possible side effects of VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is VYVGART HYTRULO® (efgartigimod alfa and hyaluronidase-qvfc)?
VYVGART HYTRULO is a prescription medicine used to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP).
It is not known if VYVGART HYTRULO is safe and effective in children.
Please see full Prescribing and Patient Information for VYVGART HYTRULO.
Dosage forms and strengths:
VYVGART Hytrulo is available as a single-dose subcutaneous injection containing: 200 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per prefilled syringe, or 180 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per vial.
IMPORTANT SAFETY INFORMATION
Do not take VYVGART HYTRULO if you are allergic to efgartigimod alfa, hyaluronidase, or any of the ingredients in VYVGART HYTRULO. VYVGART HYTRULO can cause serious allergic reactions and a decrease in blood pressure leading to fainting.
Before taking VYVGART HYTRULO, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection or fever.
- have recently received or are scheduled to receive any vaccinations.
- have any history of allergic reactions.
- have kidney (renal) problems.
- are pregnant or plan to become pregnant. It is not known whether VYVGART HYTRULO will harm your unborn baby.
- Pregnancy Exposure Registry. There is a pregnancy exposure registry for women who use VYVGART HYTRULO during pregnancy. The purpose of this registry is to collect information about your health and your baby. Your healthcare provider can enroll you in this registry. You may also enroll yourself or get more information about the registry by calling 1-855-272-6524 or going to VYVGARTPregnancy.com
- are breastfeeding or plan to breastfeed. It is not known if VYVGART HYTRULO passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
VYVGART HYTRULO can cause side effects which can be serious, including:
- Infection. VYVGART HYTRULO may increase the risk of infection. If you have an active infection, your healthcare provider should delay your treatment with VYVGART HYTRULO until your infection is gone. Tell your healthcare provider right away if you get any of the following signs and symptoms of an infection:
- fever
- chills
- frequent and painful urination
- cough
- pain and blockage of nasal passages
- wheezing
- shortness of breath
- sore throat
- excess phlegm
- nasal discharge
- Allergic reactions (hypersensitivity reactions). VYVGART HYTRULO can cause allergic reactions that can be severe. These reactions can happen during, shortly after, or weeks after your VYVGART HYTRULO injection. Tell your healthcare provider or get emergency help right away if you have any of the following symptoms of an allergic reaction:
- rash
- swelling of the face, lips, throat, or tongue
- shortness of breath
- hives
- trouble breathing
- low blood pressure
- fainting
- Infusion or injection-related reactions. VYVGART HYTRULO can cause infusion or injection-related reactions. These reactions can happen during or shortly after your VYVGART HYTRULO injection. Tell your healthcare provider if you have any of the following symptoms of an infusion or injection-related reaction:
- high blood pressure
- chills
- shivering
- chest, stomach, or back pain
The most common side effects of VYVGART HYTRULO include respiratory tract infection, headache, urinary tract infection, and injection site reactions.
These are not all the possible side effects of VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What is VYVGART HYTRULO® (efgartigimod alfa and hyaluronidase-qvfc)?
VYVGART HYTRULO is a prescription medicine used to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP).
It is not known if VYVGART HYTRULO is safe and effective in children.
Please see full Prescribing and Patient Information for VYVGART HYTRULO.
Dosage forms and strengths:
VYVGART Hytrulo is available as a single-dose subcutaneous injection containing: 200 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per prefilled syringe, or 180 mg/mL of efgartigimod alfa and 2,000 U/mL of hyaluronidase per vial.